VEEVA VAULT CLINICAL
Transform Clinical Development
Streamline clinical trials from study start-up to close.
Veeva Vault Clinical
Vault Clinical transforms clinical operations and clinical data management with the most comprehensive suite of clinical solutions, offering EDC, coding, data management, study start-up, eTMF, CTMS, and payments on a single cloud platform. Life sciences companies can increase visibility, streamline end-to-end processes, and improve how sponsors, CROs, and sites work together throughout the clinical trial process.
Vault eTMF
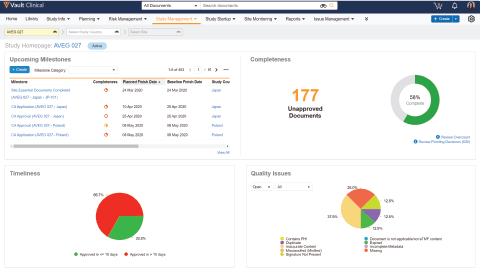
Vault eTMF enables active TMF management for real-time inspection readiness, visibility, and control.
Learn moreVault CTMS
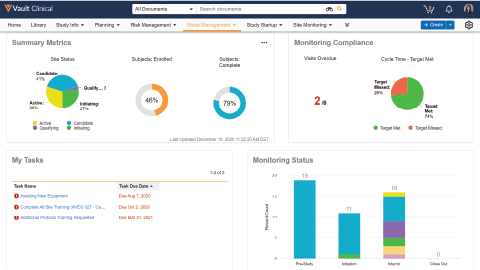
Vault CTMS unifies clinical information, documentation, and processes globally to reduce complexity, increase transparency, and speed time to critical decision making.
Learn moreVeeva Site Connect
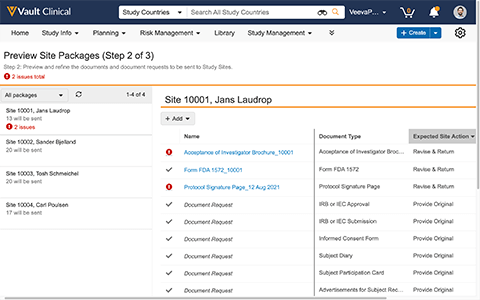
Veeva Site Connect automates information sharing between Vault Clinical applications and Veeva SiteVault, a compliant eISF application used by sites.
Learn moreVault Payments
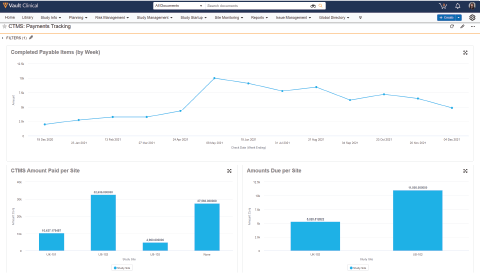
Vault Payments speeds payments to clinical research sites and provides financial visibility to all study partners.
Learn moreVault Study Startup
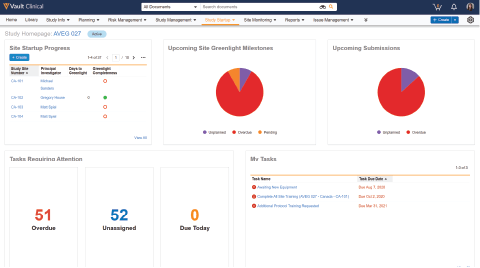
Vault Study Startup accelerates time to site activation by bringing together start-up content and data, and incorporating country-specific intelligence in study start-up workflows.
Learn moreVault Study Training
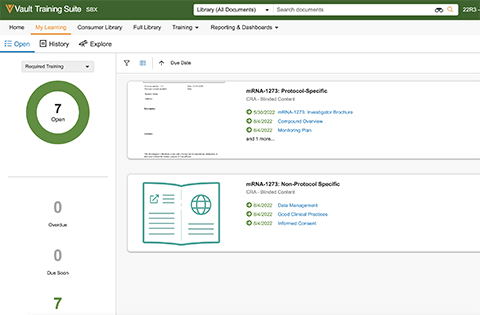
Veeva Vault Study Training brings sponsors, CROs, and research sites into a single platform to streamline and automate training while ensuring Vault eTMF is inspection ready.
Learn moreVeeva eConsent
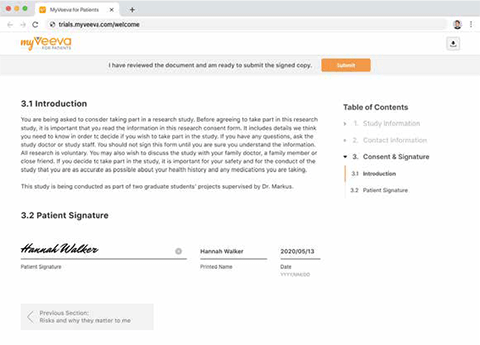
Veeva eConsent simplifies the set-up, completion, and review of informed consent through an end-to-end process that reduces administrative burden and delivers a better experience for sites and patients.
Learn moreVault EDC
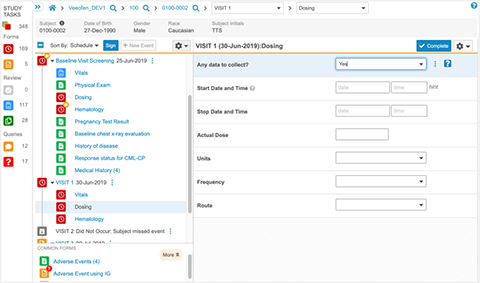
Vault EDC accelerates study cycle times with faster builds, easy amendments, intuitive data capture, and next-generation monitoring and data review.
Learn moreVeeva CDB
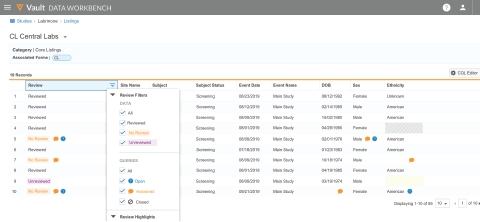
Veeva CDB is a central environment for aggregating, cleaning, and transforming clinical data from multiple data sources.
Learn moreVeeva RTSM
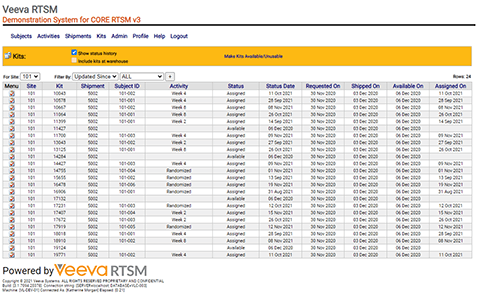
Veeva RTSM supports multiple different randomization schemas to handle your most complex study designs. Flexible control over trial supply with advanced tools will minimize drug wastage.
Learn moreVeeva ePRO
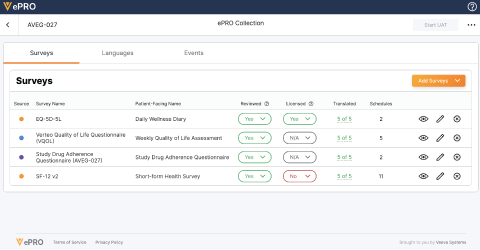
Veeva ePRO increases efficiencies through integrated workflows that enable end-to-end management of ePRO solutions from creation through data capture to reporting.
Learn moreTransform Clinical Operations and Data Management
Streamline clinical trials from study start-up to close
cut in TMF reconciliation time with Vault eTMF
reduction in monitoring visit preparation time with Vault Unified Clinical Operations
savings per average phase III trial with Vault CTMS
Bringing Agility, Visibility, and Speed to Clinical Trials
Companies around the world are using Veeva to help improve trial performance





Resources for Vault Clinical